Arxius de Miscel·lània Zoològica. Volumen 20 (2022) Páginas: 13-25
New finds of naked amoebae in the Black Sea (Ukraine)
DOI: https://doi.org/10.32800/amz.2022.20.0013Descargar
PDFCita
Patsyuk, M., 2022. New finds of naked amoebae in the Black Sea (Ukraine). Arxius de Miscel·lània Zoològica, 20: 13-25, DOI: https://doi.org/10.32800/amz.2022.20.0013-
Fecha de recepción:
- 17/02/2022
-
Fecha de aceptación:
- 03/05/2022
-
Fecha de publicación:
- 16/05/2022
-
Compartir
-


-
Visitas
- 2918
-
Descargas
- 1329
Abstract
New finds of naked amoebae in the Black Sea (Ukraine)
Our research identified 12 species of naked amoebae of eight morphotypes in the Black Sea (coastal zone of Zatoka village, Odessa region). In more than half of the samples, Vannella devonica, Vannella aberdonica, and Thecamoeba orbis were present. The species Saccamoeba marina, Vexillifera armata, Vannella plurinucleolus, Cochliopodium gulosum, and Stenamoeba sp. were rare and infrequent in our samples. The upper layers of the bottom sediment were inhabited by all 12 species of amoebae; two layers were inhabited by S. marina, V. armata, V. devonica, V. aberdonica, Mayorella gemmifera, T. orbis, Stenamoeba sp., Acanthamoeba griffini; three layers were colonized by V. armata, V. devonica, V. aberdonica, T. orbis, Stenamoeba sp., and A. griffini. All amoebae species and their morphotypes occurred at Black Sea water temperatures of + 22ºC to + 26ºC and salinity of 15.5‰ to 17.6‰. The species V. simplex and A. griffini, which belong to the fan-shaped and acanthopodial morphotypes, respectively, were collected in samples from the Mediterranean Sea at water temperatures of + 29ºC and salinity of 37.8‰. The amoebas identified belong to two classes, seven orders, eight families and eight genera.
Dataset published through Zenodo (Doi: 10.5281/zenodo.6557376)
Key words: Naked amoebae, Morphotypes, Bottom sediment, The Black Sea, Water salinity
Resumen
Nuevos hallazgos de ameba desnuda en el mar Negro (Ucrania)
Nuestra investigación identificó 12 especies de ameba desnuda de ocho morfotipos en el mar Negro (zona costera de Zatoka, un pueblo de la región de Odesa). En más de la mitad de las muestras había presencia de Vannella devonica, Vannella aberdonica y Thecamoeba orbis. La presencia de las especies Saccamoeba marina, Vexillifera armata, Vannella plurinucleolus, Cochliopodium gulosum y Stenamoeba sp. fue rara y poco frecuente en nuestras muestras. Los estratos superiores del sedimento del fondo marino están habitados por las 12 especies de ameba; dos estratos están habitados por S. marina, V. armata, V. devonica, V. aberdonica, Mayorella gemmifera, T. orbis, Stenamoeba sp. y Acanthamoeba griffini, y tres estratos están colonizados por V. armata, V. devonica, V. aberdonica, T. orbis, Stenamoeba sp. y A. griffini. Todas las especies de ameba y sus morfotipos se encontraron a las temperaturas y salinidad del mar Negro desde + 22ºC a +26ºC y de 15,5‰ a 17,6‰, respectivamente. Las especies V. simplex y A. griffini, que pertenecen a los morfotipos flabelado y acantopodial, respectivamente, fueron recolectadas en muestras del mar Mediterráneo a una temperatura del agua de +29ºC y una salinidad del 37,8‰. Las amebas identificadas pertenecen a dos clases, siete órdenes, ocho familias y ocho géneros.
Datos publicados en Zenodo (Doi: 10.5281/zenodo.6557376)
Palabras clave: Ameba desnuda, Morfotipos, Sedimentos del fondo, Mar Negro, Salinidad del agua
Resum
Noves troballes d’ameba nua al mar Negre (Ucraïna)
La nostra recerca va identificar 12 espècies d’ameba nua de vuit morfotips al mar Negre (zona costanera de Zatoka, un poble de la regió d’Odessa). En més de la meitat de les mostres hi havia presència de Vannella devonica, Vannella aberdonica i Thecamoeba orbis. La presència de les espècies Saccamoeba marina, Vexillifera armata, Vannella plurinucleolus, Cochliopodium gulosum i Stenamoeba sp. va ser rara i poc freqüent a les nostres mostres. Els estrats superiors del sediment del fons marí estan habitats per les 12 espècies d’ameba; dos estrats estan habitats per S. marina, V. armata, V. devonica, V. aberdonica, Mayorella gemmifera, T. orbis, Stenamoeba sp. i Acanthamoeba griffini, i tres estrats estan colonitzats per V. armata, V. devonica, V. aberdonica, T. orbis, Stenamoeba sp. i A. griffini. Totes les espècies d’ameba i els seus morfotips es van trobar a les temperatures i salinitat del mar Negre des de +22ºC a +26ºC i de 15,5‰ a 17,6‰, respectivament. Les espècies V. simplex i A. griffini, que pertanyen als morfotips flabel•lat i acantopodial, respectivament, van ser recol•lectades en mostres del mar Mediterrani a una temperatura de l’aigua de +29ºC i una salinitat del 37,8‰. Les amebes identificades pertanyen a dues classes, set ordres, vuit famílies i vuit gèneres.
Dades publicades a Zenodo (Doi: 10.5281/zenodo.6557376)
Paraules clau: Ameba nua, Morfotips, Sediments del fons, Mar Negre, Salinitat de l’aigua
Introduction
Naked amoebae are protists that inhabit various natural habitats, including fresh, marine and brackish water bodies, and soils. Numerous works are devoted to the study of marine amoebae. Studying the fauna of the Mediterranean Sea, Dujardin (1841) discovered the first marine amoeba-like organisms. Later, Schaeffer described a large number of marine amoebas (Schaeffer, 1926). These animal-like organisms are mentioned in the studies of Biernacka (1963) and Kufferath (1952), who studied the fauna of Gdansk Bay and the North Sea, respectively. Targeted studies to identify new species and genera of marine amoebae have been conducted by authors such as Sawyer (Sawyer, 1971a, 1971b, 1975a, 1975b, 1980), Page (Page, 1979), Rogerson (Rogerson and Hauer, 2002), and Moran (Moran et al., 2007). Page conducted a number of studies on the isolation of marine amoebae from samples in the United States (Atlantic coast), Great Britain, the Persian Gulf, and Australia (Page, 1970, 1971a, 1971b, 1973, 1974, 1976, 1979, 1983). Based on the results of these large-scale studies, he published a key to identify marine amoebae (Page, 1983). Previously, the first identification key of marine amoebae was published by Bovee and Sawyer (Bovee and Sawyer, 1979). Anderson and Rogerson morphologically described a large number of marine amoebae, and clarified their biotope preferences and the influence of environmental factors on their distribution (Rogerson, 1991; Rogerson and Laybourn–Parry, 1992; Anderson and Rogerson, 1995; Anderson et al., 1997; Rogerson and Gwaltney, 2000). Most of the above studies are based on light and electron microscopy.
With the development of molecular genetic research methods, the system of naked amoebae has undergone significant changes. Most taxa have been reviewed and clarified. New taxa of these organisms have been described, and changes and refinements have been made regarding the the names of a several naked amoebae (Cavalier-Smith et al., 2004, 2015, 2016; Dykova et al., 2008, 2011; Tekle et al., 2008; Kang et al., 2017).
We conducted research on the species composition of naked amoebae in fresh water and soils in the Ukraine (Patsyuk, 2014, 2019, 2020). We identified a total of 44 freshwater and 23 soil amoeba species. The composition of amoebae in the marine fauna of Ukraine is unknown. For the first time, we conducted studies of species composition of these protists in the Black Sea and tried to analyze the peculiarities of their distribution.
Material and methods
Sample selection
The material was collected in Jun-August 2019 (fig. 1). During the study, we collected and analysed 415 samples of the upper layer of the bottom sediment (to a depth of 45 cm) in the Black Sea (coastal zone of Zatoka village, Odessa region).
Fig. 1. Localización de los puntos de muestreo
To compare species composition of naked amoebae from different habitats, samples were also taken from the Mediterranean Sea (Side, Turkey); twenty-five samples were taken from different layers of the bottom sediment in this reservoir. To study the peculiarities of the distribution of naked amoebae in different layers of the bottom sediment of the Black Sea, we used the method developed by Anderson (Anderson and Rogerson, 1995). Additional water samples were taken to determine the temperature (°C) and salinity (‰) of the Black Sea and Mediterranean Sea as described in Khilchevsky (2003).
Cultivation and identification of naked amoebas
Amoebae were cultured in Petri dishes with a diameter of 50 mm with non-nutrient agar (NNA), according to the method of Page (Page, 1983, 1988, 1991). NNA: 1 litre of PJ and 15 g non-nutrient agar. Prescott's and James solution (PJ): Make up three stock solutions, each with 100 ml of glass-distilled water. Stock solution A (CaCl2·2H2O: 0.433 g; KCl: 0.162 g); Stock solution B (K2HPO4: 0.512 g); Stock solution C (MgSO4·7H2O: 0.280 g). Combine 1 ml of each stock solution and 997 ml of distilled water to make 1 litre of the final dilution. The cultures of amoebae were maintained in laboratory conditions at a temperature of +20ºC and under unregulated lighting. The sample in each Petri dish was examined once every eight days using a Lomo MBR-3 light microscope. Species were identified using light microscope Axio Imager M1 (Centre for collective usage of scientific equipment 'Animalia' of the I.I. Schmalhausen Institute of Zoology NAS of Ukraine) with differential interferential contrast, selecting living cells in a drop of water on glass slides. Amoebae were identified in two stages: first their morphotype was determined, then Page's taxonomic identification key was used (1983).
The size groups of amoeba were determined using metric for size matching: < 25 μm, small species; < 25–100 μm, medium species; > 100 μm, large species.
Although some current studies report the densities of amoebae (e.g., number per L water), few provide data on the abundance of naked amoebae. Thus, in our studies we determined the frequency of occurrence (R) for each species. The frequency of occurrence of species was defined as the ratio of samples in which the species was found to the total number of studied samples (Raunkiaer, 1934). Amoebae were considered most common if R was 50% or more, averagely frequent if R ranged from 30% to 50%, and least common if R was less than 30% (Raunkiaer, 1934).
We isolated genomic DNA from Vannella simplex and Acanthamoeba griffini. Genomic DNA was isolated using the guanidine isothiocyanate method (Maniatis et al., 1982). The 18S rRNA gene was amplified using universal eukaryotic primers RibA 5'-ACCTGGTTGATCCTGCCAGT-3' and RibB 5'-TGATCCTTCTGCAGGTTCACCTAC-3' (Medlin et al., 1988). The DNA sequences obtained with the data of GenBank (GenBank) were compared using the program BLAST (NCBI) https://blast.ncbi.nlm.nih.gov/Blast.cgi). Record numbers in the GenBank database are OM522832 for Acanthamoeba griffini, collected from the Black Sea, and OM522833 for this species sampled from the Mediterranean Sea; OM403052 for Vannella simplex isolated from the Black Sea, and OM403053 for the same species from the Mediterranean Sea.
Results and discussion
Taxonomic composition of naked amoebas
We found twelve species of naked amoebae found in the bottom sediments of the Black Sea (see also dataset published through Zenodo, Doi: 10.5281/zenodo.6557376): they belonged to two classes, seven orders, eight families and eight genera, and are listed below following the taxonomic systems in Adl et al. (2012, 2019) and Page (1991). The class Tubulinea is represented by only one species (Saccamoeba marina), while the class Discosea represented the majority (92 %, 11 species).
Сlass Tubulinea Smirnov et al., 2005
Order Euamoebida Lepsi, 1960
Family Hartmannellidae Volkonsky, 1931
Genus Saccamoeba Frenzel, 1897
Saccamoeba marina Anderson, Rogerson and Hannah, 1997
Class Discosea Cavalier-Smith et al., 2004
Order Dactylopodida Smirnov et al., 2005
Family Vexilliferidae Page,1987
Genus Vexillifera Schaeffer, 1926
Vexillifera armata Page, 1979
Order Vannellida Smirnov e tal., 2005
Family Vannellidae Bovee, 1979
Genus Vannella Bovee, 1965
Vannella devonica Page, 1979
Vannella aberdonica Page, 1980
Vannella simplex Bovee, 1965
Vannella plurinucleolus (Page, 1974) Smirnov et al., 2007
Order Himatismenida Page, 1987
Family Cochliopodiidae De Saedeleer, 1934
Genus Cochliopodium Hertwig and Lesser, 1874
Cochliopodium gulosum (Schaefer, 1926) Kudryavtsev, 2000
Order Dermamoebida Cavalier-Smith, 2004
Family Mayorellidae Schaeffer, 1926
Genus Mayorella Schaeffer, 1926
Mayorella gemmifera Schaeffer, 1926
Order Thecamoebida Smirnov and Cavalier-Smith, 2011
Family Thecamoebidae Schaeffer, 1926
Genus Thecamoeba Fromentel, 1874
Thecamoeba orbis Schaeffer, 1926
Thecamoeba hilla Schaeffer, 1926
Family Stenamoebidae Cavalier-Smith, 2016
Genus Stenamoeba Smirnov, Nassonova, Chao et Cavalier-Smith, 2007
Stenamoeba sp.
Order Centramoebida Rogerson and Patterson, 2002
Family Acanthamoebidae Sawyer and Griffin, 1975
Genus Acanthamoeba Volkonsky, 1931
Acanthamoeba griffini Sawyer, 1971
Distribution of naked amoebas
Three species of naked amoebae (25 % of the 12 identified species in total) were found in more than half of the samples and were the most common in the studied water body: V. devonica (63 %), T. orbis (58 %), and V. aberdonica (56.5 %) (fig. 2).
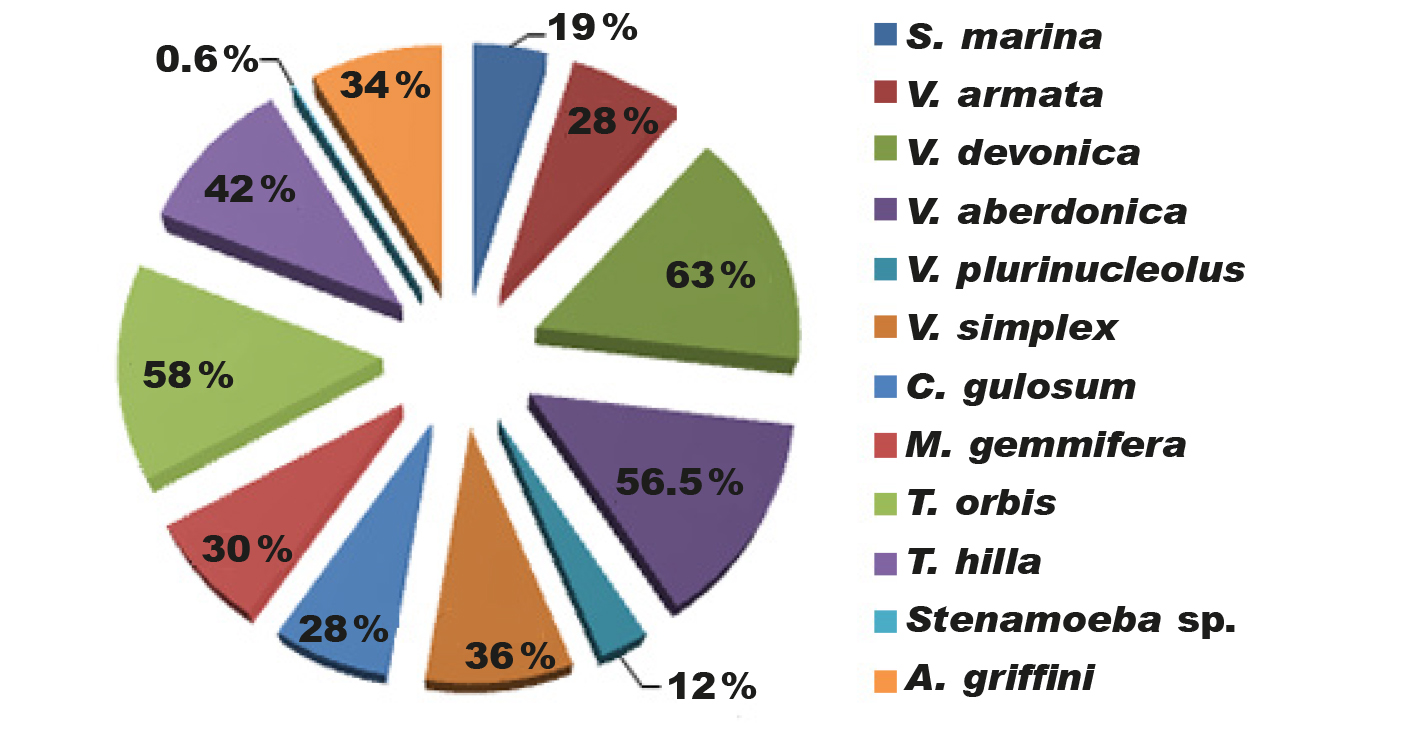
Fig. 2. Frecuencia de ocurrencia de amebas en muestras del sedimento del fondo del mar Negro (Ucrania).
Five species of naked amoebae (approximately 42% of the total number of species) were isolated only a few times during the sampling period and can be classified as rare, with few species in this water body. These species were Stenamoeba sp. (0.6%), V. plurinucleolus (12%), S. marina (19%), V. armata (28%), and C. gulosum (28%) (fig. 2). According to the metric parameters of naked amoebae (table 1), the studied reservoir was dominated by medium-sized amoebae (S. marina, V. devonica, V. simplex, V. plurinucleolus, C. gulosum, M. gemmifera, T. hilla, Stenamoeba sp., A. griffini), which is 75% of the total number of identified naked amoebae. Other amoebas found (25% of all identified species) were small (V. armata, V. aberdonica, T. orbis) (table 1). Large amoebae were not recorded in the studied reservoir during the sampling period. Among the most common amoebae in the Black Sea, two species are small and one is a medium-sized amoeba. The rare and averagely frequent species of amoebae are dominated by medium-sized amoebae. The variety of naked amoebae is known to be high within a water body. Most amoebae inhabit the upper layers of the sediment, but some species can be found in fairly deep samples. Such features of the arrangement of species by depth in the samples indicate significant heterogeneity of the microspace distribution of amoebae in the bottom sediment (Anderson and Rogerson, 1995; Butler and Rogerson, 1995).
We tried to analyze the species composition of amoebae in different layers of the bottom sediment (0–15 cm; 15–30 cm; 30–45 cm) of the studied marine water body (table 2). The amoebas were hence classified into the following 'ecological groups'.

Tabla 1. Parámetros métricos de las amebas desnudas halladas e identificadas en el mar Negro (Ucrania): M, medición de la célula, en μm.

Tabla 2. Composición y morfotipos de las especies de ameba desnuda en diferentes estratos del sedimento del fondo (LBS) del mar Negro (Ucrania).
The first group consists of amoebae that inhabit the upper layers of the bottom sediment (0-15 cm). This group includes all the amoebae we have identified (S. marina, V. armata, V. devonica, V. aberdonica, V. plurinucleolus, V. simplex, C. gulosum, M. gemmifera, T. orbis, T. hilla, Stenamoeba sp., A. griffini). These are medium-sized and small amoebae. Frequency of occurrence of amoeba species in this layer of the marine bottom sediment is as follows: A. griffini (55 %), S. marina (55 %), V. armata (50 %), Stenamoeba sp. (46 %), T. hilla (42 %), V. devonica (39 %), T. orbis (38 %), M. gemmifera (37 %), V. aberdonica (36 %), V. simplex (36 %), C. gulosum (28 %), V. plurinucleolus (12 %) (fig. 3).
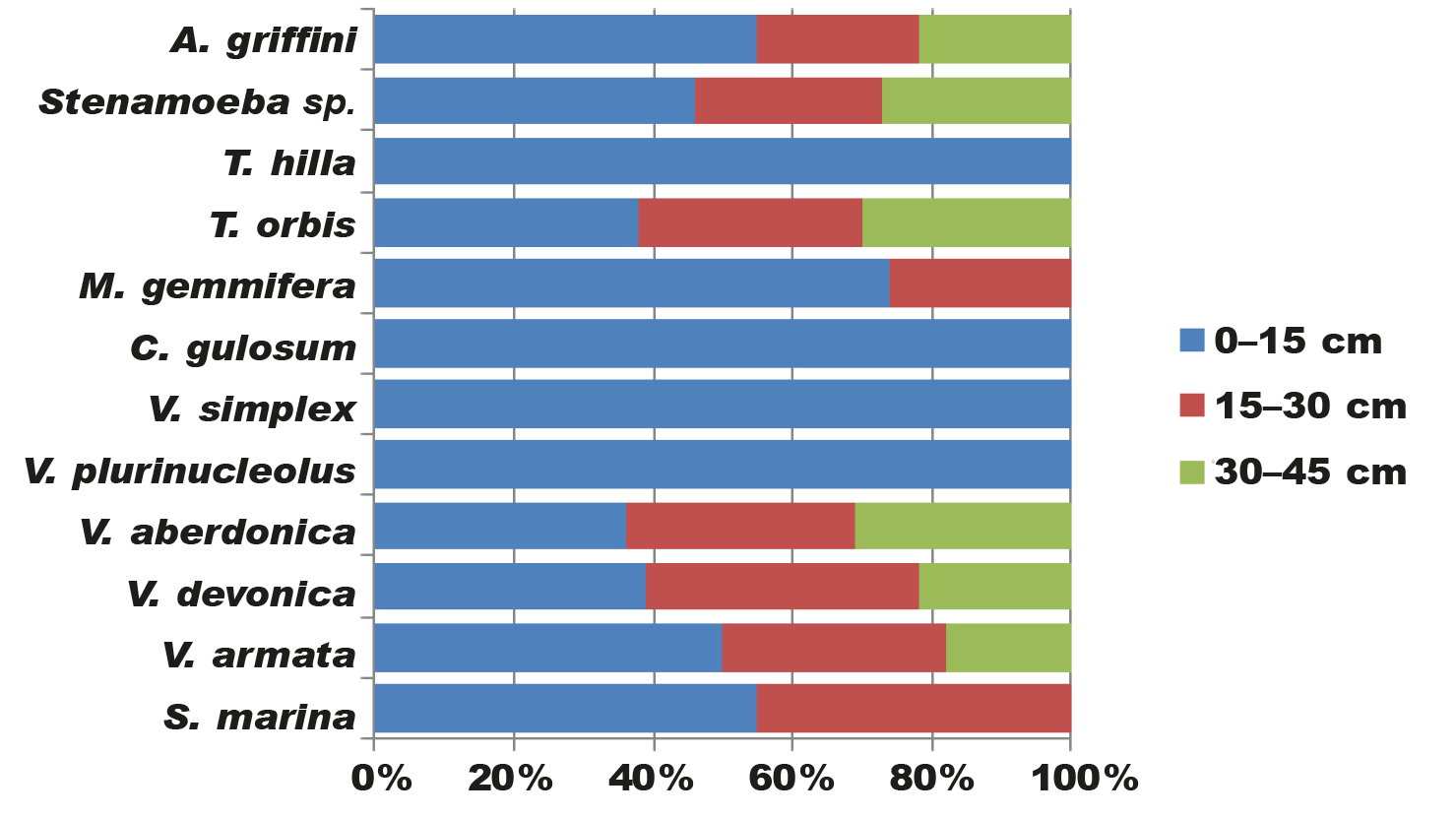
Fig. 3. Frecuencia de ocurrencia de amebas desnudas en muestras de diferentes estratos del sedimento del fondo del mar Negro (Ucrania).
The third group consists of amoebae which occur in all three layers of bottom sediment (0-15 cm, 15-30 cm and 30-45 cm) of the Black Sea. These are medium and small amoebae. This group includes six species, which is 50% of all amoebae we found: V. aberdonica (31 %), T. orbis (30 %), Stenamoeba sp. (27 %), V. devonica (22 %), A. griffini (22 %), V. armata (18 %) (fig. 3).
Naked amoebas are difficult to observe in nature. They can be detected only during reproduction in artificial conditions. Therefore, the relationship between naked amoebae and environmental factors has been little studied. There are methods to obtain species from marine habitats using accumulative cultures (Page, 1983; Dykova and Kostka, 2013). In the works on the influence of different salinity on the physiology of naked amoebae, the ranges of amoeba resistance to this factor are indicated, as determined experimentally during amoeba reproduction (Page, 1971a, 1971b, 1974, 1983; Sawyer, 1971b, 1975a, 1975b; Rogerson and Gwaltney, 2000; Cole et al., 2010). There are euryhaline and stenohaline species, as well as typical freshwater species that can be isolated from saltwater (Page, 1970; Garstecki and Arndt, 2000). However, it is believed that marine species cannot be isolated from freshwater (Page, 1988, 1991).
For a number of amoeba species, the salinity levels or ranges in which they can occur are known: 32.4 ‰ for S. marina (Anderson et al., 1997), 3.5–35 ‰ for V. plurinucleolus (Page, 1974), 3–30 ‰ for T. orbis and T. hilla (Page, 1971b), and 0–32 ‰ for A. griffini (Sawyer, 1971b).
The amoebae recorded in present study occurred in the Black Sea at water temperatures from +22 °С to +26 °С, water salinity from 15.5 ‰ to 17.6 ‰.
It should be noted that we isolated V. simplex and A. griffini from the bottom sediment of the Mediterranean Sea (Side, Turkey) at a water temperature of +29 °C and water salinity of 37.8 ‰. A. griffini occurred in all layers of the studied bottom sediment (0–15 cm; 15–30 cm; 30–45 cm), and V. simplex was found only in the upper layer of the bottom sediment (0–15 cm) of the Mediterranean Sea.
Features distribution of naked amoeba morphotypes
The amoebae we recorded belong to the monotactic, dactylopodial, fan-shaped, lens-like, mayorellian, striate, lingulate, and acanthopodial morphotypes (table 2).
The striate morphotype (50.5 %) of amoebae was the most common.The average position in terms of frequency of occurrence was occupied by fan-shaped (40 %), acanthopodial (34 %), and mayorellian (30 %) morphotypes of naked amoebae. The dactylopodial (28 %), lens-like (28 %), monotactic (19 %), and lingulate (0.6 %) morphotypes of naked amoebae were rare.
Regarding the distribution of morphotypes of naked amoebae in different layers of the bottom sediment of the Black Sea, dactylopodial, fan-shaped, striate, lingulate, and acanthopodial morphotypes of amoebae occurred in all layers (0–15 cm; 15–30 cm; 30–45 cm); two layers (0–15 cm; 15–30 cm) of the bottom sediment were occupied by monotactic and mayorellian morphotypes of naked amoebae; the lens-like morphotype of amoebae was noted in the first layer (0–15 cm) of the bottom sediment (table 2).
The frequency of amoeba morphotypes in the first layer of the bottom sediment was: monotactic 55 %, acanthopodial 55 %, dactylopodial 50 %, lingulate 46 %, striate 39 %, mayorellian 37 %, fan-shaped 31 %, and lens-like 28 %. In the second layer of the bottom sediment, the frequency of amoeba morphotypes was as follows: monotactic in 45 %, fan-shaped in 35 %, dactylopodial in 32 %, striate in 32 %, lingulate in 27 %, acanthopodial in 23 %, and mayorellian in 13%. In the third layer of the bottom sediment, the frequency was 30 % for the amoeba of striate morphotype, 27 % for those of lingulate morphotype, 24 % for the fan-shaped morphotype, 22 % for the acanthopodial morphotype, and 18 % for the dactylopodial morphotype (fig. 4).

Fig. 4. Frequency of occurrence of the morphotypes of naked amoebae in samples of the bottom sediment layers of the Black Sea (Ukraine).
The morphotypes and species of naked amoebae were recorded in the Black Sea at water temperatures ranging from +2 2°C to +26 °C, and water salinity of 15.5 ‰ to 17.6 ‰. In addition, we observed the fan-shaped morphotype of the naked amoeba, to which V. simplex belongs, and the acanthopodial morphotype of the amoeba, to which A. griffini belongs, in the Mediterranean Sea at water temperatures of +29 °C and salinity of 37.8 ‰.
Conclusion
We identified 12 species of naked amoebae belonging to eight morphotypes in the Black Sea. Three species of amoebae were commonly found, four species were of average occurrence, and five species were rare. Given the metric characteristics of the naked amoeba, we found that Black Sea fauna is dominated by medium-sized amoebae, while the large amoebae are absent.
All amoebae identified in our study inhabit the upper layers (0–15 cm) of the bottom sediment, eight amoeba species were found in two layers (0–15 cm and 15–30 cm), and six species were found in three layers (0–15 cm; 15–30 cm; 30–45 cm). The dactylopodial, fan-shaped, striate, lingulate and acanthopodial morphotypes of amoebae occurred in all layers of the bottom sediment of the Black Sea. The monotactic and mayorellian morphotypes of naked amoebae were noted in two layers of sediment. The lens-like morphotype of amoebae was observed only in the first layer of sediment.
The amoebas and their morphotypes were found in Black Sea water temperatures ranging from + 22 °С to + 26 °С, with salinity ranging from 15.5 ‰ to 17.6 ‰. We isolated V. simplex, of the fan-shaped morphotype, and A. griffini, which belongs to the acanthopodial morphotype, from the Mediterranean Sea at a water temperature of + 29 °С and water salinity of 37.8 ‰.



















